Erasmus Plus Call 2021 Round 1 KA2 funded by EU KA220-SCH
Cooperation partnerships in school education
Let’s Save Our Environment and Our Future
Project number: 2021-1-DE03-KA220-SCH-000023948
Handbook
Kit 5-10 age
Author: CSFNSM
Ver 1.0

Title: Handbook KIT 5-10 age Manuscript completed in June 2024
Erasmus+ Project no: KA220-SCH
Let’s Save Our Environment and Our Future Project number:
COPYRIGHT NOTICE
“Unless otherwise noted, the reuse of this document is authorised under the Creative Commons Attribution 4.0 International (CC BY 4.0) licence (https://creativecommons.org/licenses/by-nc-sa/4.0/). This means that reuse is allowed, provided that appropriate credit is given and any changes are indicated”.
Attribution- NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0) You are free to:
Share — copy and redistribute the material in any medium or format
Adapt — remix, transform, and build upon the material
The licensor cannot revoke these freedoms as long as you follow the license terms.
Under the following terms:
Attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
NonCommercial — You may not use the material for commercial purposes.
ShareAlike — If you remix, transform, or build upon the material, you must distribute your contributions under the same license as the original.
No additional restrictions — You may not apply legal terms or technological measures that legally restrict others from doing anything the license permits.

We live together on the Earth planet: a wonderful mixture of mountains, seas, oceans, plants, animals, etc. However, with the growing of industrial activities and the expansion of urbanization, most of our planet resources are in dangerous: forests are disappearing, the quality of air is becoming worse, water is greatly polluted, especially near the city.
Environmental protection is responsibility of Government as well of every citizen of all age. Everyone should make effort to protect the environment. Putting in place Environmental Protection Measures means organize actions to effect Environmental Quality, including but not limited to, assessment and prediction of impacts, monitoring, measure to avoid or mitigate impacts, setting of limits for environmental degradation, etc.
But what kind of things are we capable of to protect the environment?
Education is the first measurement that we can do: creating awareness of problems is the first step in trying to solve them.
To begin let’s try to know nature better by measuring the parameters and quantities that characterize it, such as temperature, humidity, atmospheric pressure, water quality, light, color, light and so on…
KIT 5-10
Materials Needed
Thermometer
4+ Cups
Pipes
Litmus paper “Tornasole” strips
Water and liquid samples
Soil samples
Baking soda
Vinegar
Candle
Chalks
Elastics
Dishes and containers
Different solid objects
Colored cardboard
Scissors
1 CD disk
Black/white color
Notebook or paper
Pen or pencil
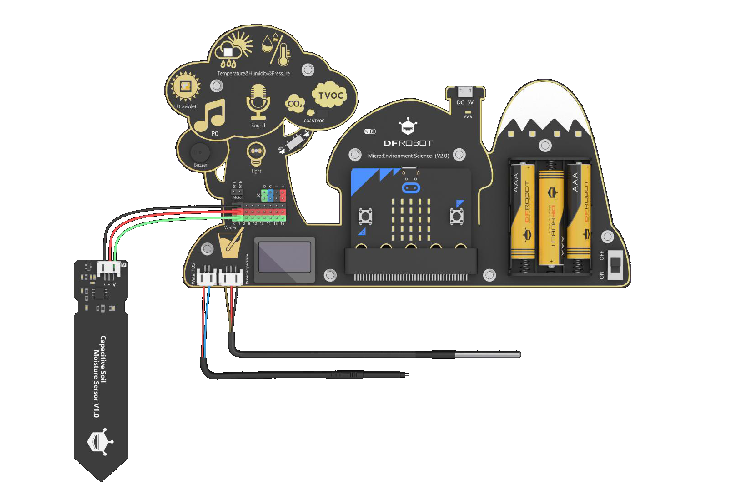
You may also use the Electronic Board: Environment Science Expansion Board for micro:bit -V2.0 (SKU: MBT0034) described in Appendix. You may also refer to the 11-17 HandBook for code descriptions and details to operate the board.

Among many environmental parameters, temperature and humidity are the ones which mainly impacts on human health and comfort.
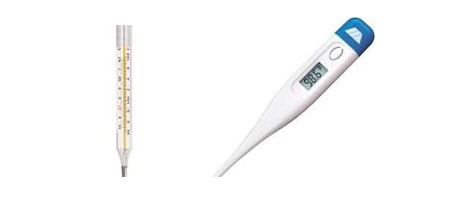
5-10 Kit contains a mercury-in-glass thermometer. Introduce the concept of temperature and thermometer, show to the kids the graduate scale of the thermometer and measure with them the temperature of different objects.
When you choose an outfit for the day, you think about it. When you touch a pan with piping hot pizza rolls on it, you experience it. When it’s high enough in your body, you’re probably sick. So what is it?
Temperature is how much heat is in something, and you experience it every day. Another way to think of temperature is how hot or cold something is. Temperatures can range from freezing cold to stifling heat. Being able to measure and understand temperature is important to many things in our lives.
Temperature is hence a measure of heat. Heat is energy, so the more energy, the higher is the temperature. You can explain to the kids by saying that if they do gym they will spend energy (kinetic energy) and they become warmer.
Units: Currently the most common temperature units are: Celsius (C) and Fahrenheit (F)
Temperature in Europe is measured in Celsius degree: Celsius scale is based on the freezing point of the water being 0 degrees and the boiling point being 100 degrees.
You may wish or not (depending on the grade of your class) to explain conversion between Celsius and Fahrenheit.
Temperature Measurements
The most common device to measure the temperature of a system is a thermometer. The most common thermometers are the “bulb-ones”, which usually are mercury-in-glass thermometers or digital thermometers base on infrared.
Activity
- First, examine your thermometer and determine what the units are (in Europe should be Celsius).
- Fill 4 cups of water to the same volume, ensuring it is enough to submerge your thermometer. Record the temperature of each cup of water by submerging the thermometer in the water until the temperature stabilizes.
- Leave one cup of water at room temperature. Place one cup outside for an hour. Heat one cup of water in the microwave for 2 minutes and place one cup of water in the freezer for 1 hour.
- Record the temperature of each cup of water after the time specified above has passed.
- Did the temperature change for the water in each cup? If so, did it increase or decrease? Explain why the temperature changed for each instance.
- Comment about the temperature of the outside sample. This is linked to the weather condition: is it a sunny or cloudy day? What do you expect: increase or decrease? Why?
- Besides the weather, what are some other applications for temperature?
Discussion
The cup of water at room temperature is not expected to change, assuming the temperature in the room does not vary. The cup that was heated in the microwave should exhibit an increase in temperature since the water is being heated. It is expected that the cup of water in the freezer would decrease since the refrigerator is colder than room temperature. Finally, the cup of water placed outside may exhibit an increase or decrease in temperature based on the weather outside. It would be useful to take the temperature of the outdoors and compare it to the one of the cup of water to see how much of a difference there is.
It’s too hard to control temperature and humidity in the atmosphere, but it will not be hard to control the indoor temp and humidity with equipment such as air conditioners, humidifiers, electric fans and so on. Comment about the environmental conditions.
Further insight:
According to national legal provisions and those dictated by the WHO (the World Health Organization), the ideal temperature to keep in the home must be around 20° C, with a maximum fluctuation of 2° above or below.
The humidity rate, however, must be around 40-50%. More specifically, during the winter, having a good level of humidity helps to avoid dryness of the mucous membranes of the respiratory system. In summer, however, if you use the air conditioner, you need to dehumidify the environment, since it is precisely the humidity that is too high that makes you feel muggier. The ultra-low humidity will lead to excessive drying, one is easy to produce static electricity and increase the density of dust easily, so 50%-60% humidity is appropriate. On the contrary, an environment with too high humidity
These values of course depend also on the season and on the specific climate of region. Generally, the human body feel comfortable when the temperature is between 17-28 degrees and the humidity between 50%-60%.
Temperature measurements are used in many different applications. For example, while cooking, meats and other foods need to reach a certain temperature for safety. Food thermometers can be used to ensure the food has been thoroughly heated throughout. Temperatures are also taken at the doctor to determine if a patient may have a fever. Temperature sensors are also incorporated into electronics, such as our refrigerators, to ensure food is stored appropriately.
Select now a set of solid objects and let the children touch them and order them accordingly their feeling of cold and hot. Annotate that into a notebook and then measure their temperature. You will experience that by using a bulb thermometer is more difficult to measure the solid temperature than the liquid one.
Introduce the digital thermometer (you just need one per class).
What about the temperature display in the car or that thing that the doctor puts in my ear that beeps? Those are called digital thermometers. They have electric sensors that read the temperature and send the information to a tiny computer that displays the temperature.
Try to measure the temperature of 4-5 different objects by using the two types of thermometers and compare the results.
In the age of the Internet of Things, we also use a device called a “temperature sensor” to measure temperature which can display the measured data on a screen or to a computer for analysis. Smaller and easier to use.
Without going into the details, you can show the Environmental Electronic Board and explain that this board is equipped with many objects (sensors) able to measure different environmental parameters such as the temperature. You may prepare in advance the Board and the code needed to read-out it (refer to the Appendix and to the 11-17 Handbook for details) and let the children read the temperature looking at the OLED display.
Follow the instruction on the Appendix to know how to use the board and upload the file named microbit-Temperature.hex.
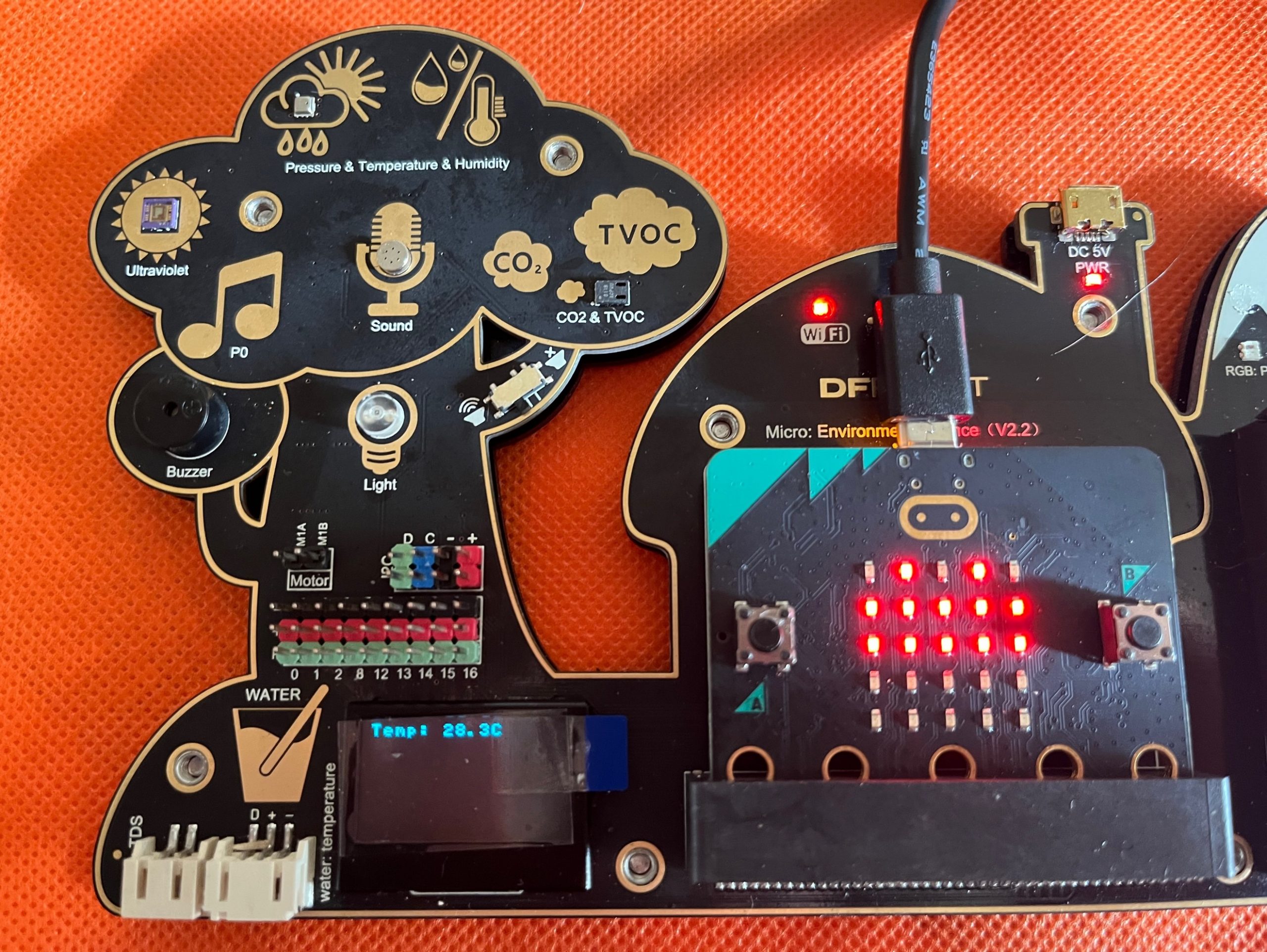
The environmental temperature is printed out on the OLED screen with the notation:
Temp: value in oC
If the temperature is lower than 15 oC or greater than 28 oC the program is instructed to print a “X” on the micro:bit board and to raise an alarm, while if the temperature is in between 15 oC and 28 oC a “Heart” is shown, to show up that the value is fine for the human working condition.
Compare the results obtained with the standard thermometer and the sensor on the electronic board. Do you experience difference? Try to interpret the results.
Let the students put their finger on the sensor surface. What happens to the temperature on the OLED?
Humidity measurement:
Have you ever felt the air just outside of a hot bath or shower? It feels wet, but you cannot see any water in the air. There really is water in the air, but it’s in the form of a gas. This kind of water is called water vapor. You can’t see it, but you can feel it. The water vapor that is in the air is called humidity. So, humidity is the amount of water vapor in the air. Water vapor is generated by evaporation of water in the Earth (lakes, rivers, etc.). Also, humans and animals produce water vapor when they breathe.
If there is a lot of water vapor in the air, the humidity will be high. The higher the humidity, the wetter it feels outside.
Humidity, as well as temperature, is strictly related to weather condition and climate.
On the weather reports, humidity is usually explained as relative humidity. Relative humidity is the amount of water vapor in the air, expressed as a percentage of the maximum amount of water vapor the air can hold at the same temperature. Think of the air at a chilly -10 degrees Celsius (14 degrees Fahrenheit). At that temperature, the air can hold, at most, 2.2 grams of water per cubic meter. So, if there are 2.2 grams of water per cubic meter when it’s -10 degrees Celsius outside, we’re at an uncomfortable 100 percent relative humidity. If there was 1.1 grams of water in the air at -10 degrees Celsius, we’re at 50 percent relative humidity.
When humidity is high, the air is so clogged with water vapor that there isn’t room for much else. If you sweat when it’s humid, it can be hard to cool off because your sweat can’t evaporate into the air like it needs to. An excess of humidity can also cause damage to electronic equipment. Moisture from humid air settles, or condenses, on electronics. This can interrupt the electric current, causing a loss of power. Computers and television sets can lose power like this if not protected from the effects of humidity. Living with humidity is easier with the aid of a dehumidifier, which sucks moisture out of the air.
High humidity is also associated with hurricanes. Air with high moisture content is necessary for a hurricane to develop. U.S. states such as Texas and Louisiana, which border the very warm Gulf of Mexico, have humid climates. This results in tons of rainfall, lots of flooding and the occasional hurricane.
However, even an excessively dry climate is not ideal for humans and normally a humidity between 30-60% is appropriate enough.
Measurement of the relative humidity
In our electronic board the same sensor used to measure the temperature could also provide the relative humidity. However, we need to use a different code to get the %humidity value.
Please Import the file
microbit-Temperature-Humidity.hex
And download on the electronic board. The code will show the temperature and relative humidity on the OLED and if the values are outside the ideal thresholds for human the microbit card will show a “X”, while if the values are good enough the card will show a “heart”
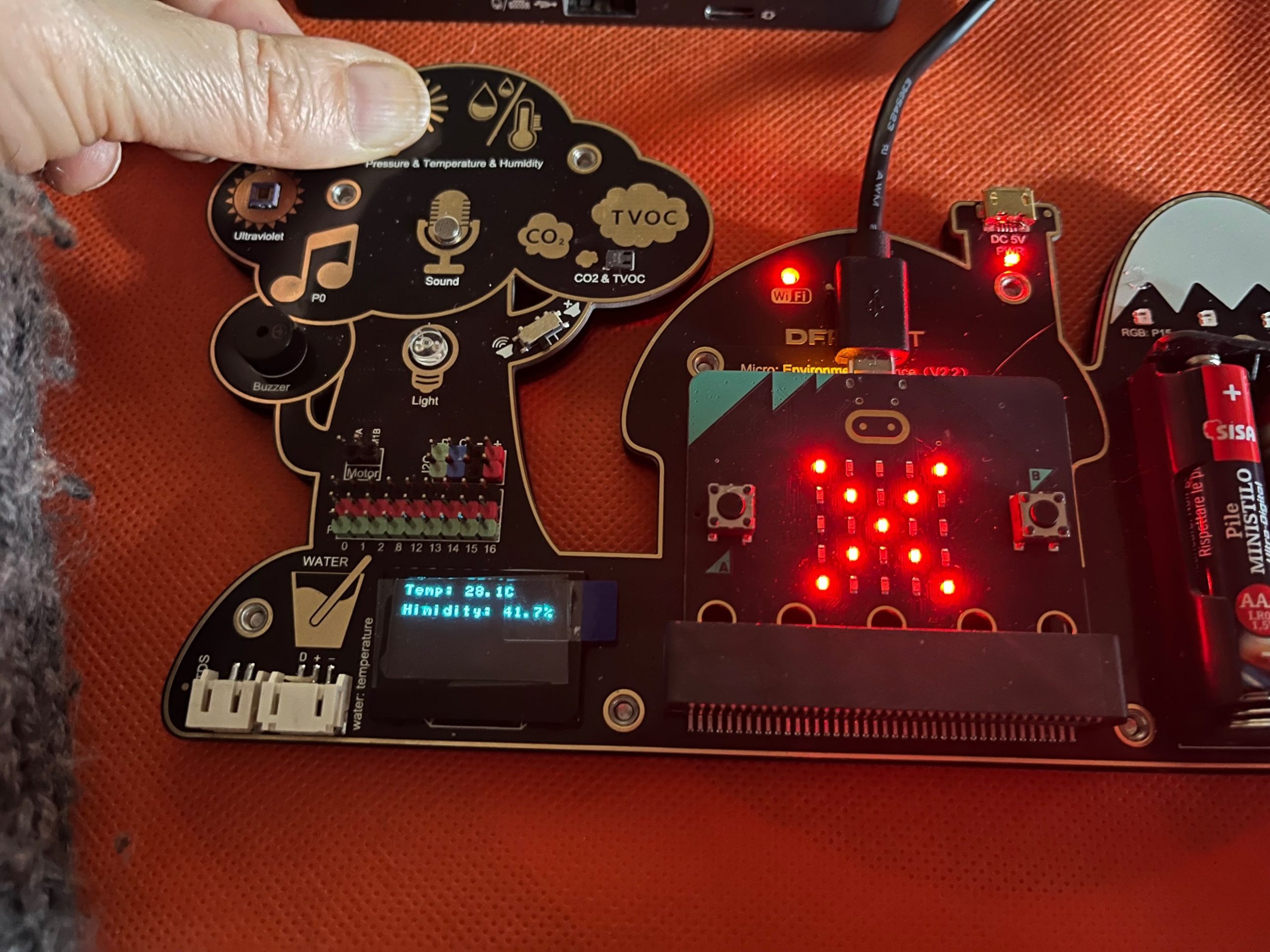
High temperature – normal humidity

Normal temperature – high humidity
Let the students place their finger on the sensor (this will raise the temperature) or breathe near the sensor (this will increase the relative humidity).
What we learned so far
Through the learning above, we have learned the following: Learned the effects of temperature and humidity on human comfort. Mastered the current temperature and humidity measurement methods. We measured the temperature and humidity around us through programming, and experienced the sensor’s measurement.

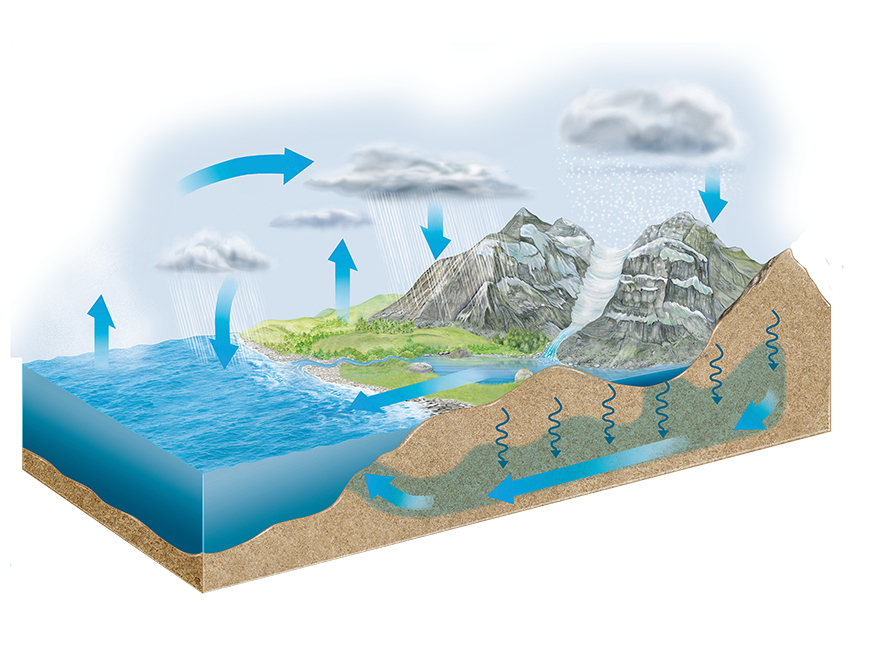
The hydrosphere is the set of all the waters on Earth. It includes the oceans and seas and continental waters (lakes, rivers, glaciers and groundwater). A small volume of water is also present in the atmosphere
Water can be solid,liquid or gas.
Water becomes denser as it cools, but only until it reaches 4°C, at which temperature it is at its maximum density (almost 1 g/cm3).
At 0 °C the density of ice is 0.99984 g/cm3 and this causes the ice to float on slightly less cold liquid water.
The properties of water are strictly related to its chemical composition and structure.

he water molecule consists of one oxygen atom (O) and two hydrogen atoms (H).
The chemical formula is H2O
Try to build a model of a water molecule with balls and toothpicks.
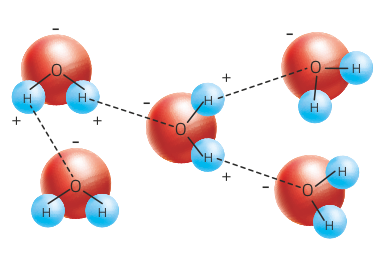
At all times on our planet there are changes of state that allow a continuous exchange of water between the land, the sea and the atmosphere. All of these exchanges constitute the hydrological cycle or water cycle.
Sea and oceans are the largest reservoir of water. However, sea water is a mixture of water and substances:
- salts of different types;
- gases in solution;
- solid particles in suspension.
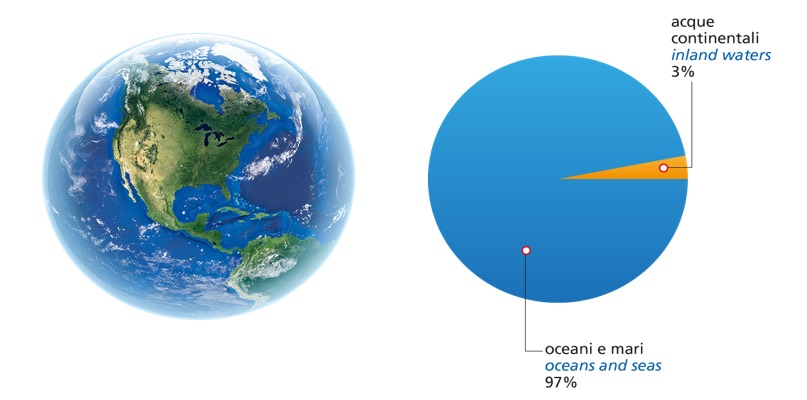
All the waters of the hydrosphere that are not found in the sea but on land (rivers, lakes, glaciers and underground aquifers) are called continental waters.
Continental waters are only 3% of the hydrosphere and represent the main reserve of fresh water.
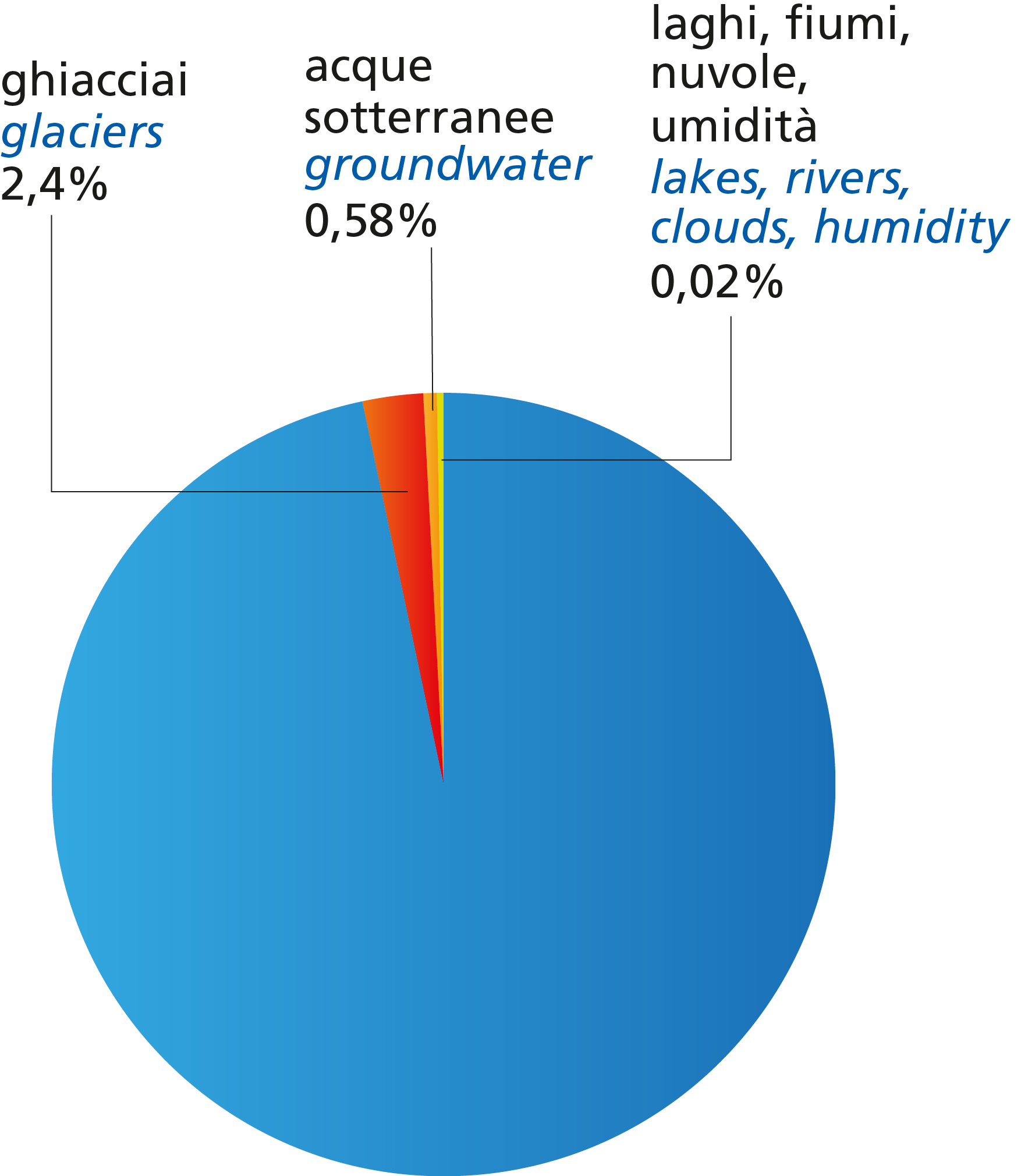
The major reservoirs of fresh water in the hydrosphere are glaciers. The largest of our planet are found in the polar regions, where they are called ice caps. The other terrestrial glaciers are found on the main mountain ranges: the Himalayas, the Andes and the Rocky Mountains. There are small glaciers in the Alps today. This means that indeed only about 1% is available as drinkable water.
The largest consumers of fresh water are:
- Agriculture: more than70% of freshwater is used for agriculture purpose. The amount of water used varies from place to place and depends on factors such as the type of food being produced, local climate conditions (the temperature and how often it rains) and the irrigation systems used.
- Industry and energy: after agriculture, industry is the second largest user of water, accounting for 22 percent of global usage. Water is used for many industrial purposes, such as cooling, cleaning, heating, generating steam, and transporting dissolved substances or particles. In addition, water is an essential part of many products (like drinks, cosmetics, or medicines). While the volume of water for industrial use is relatively low overall, industry affects water availability through pollution. A lot of industrial waste is discharged into open water sources, harming the quality of large volumes of water.
- Domestic use: There’s water for drinking, of course: humans need a minimum of 2 litres of drinking water per day to survive. In addition, we need water for cooking, cleaning, washing and sanitation. Globally, domestic use accounts for 8 percent of the water used by humans.
Population, urbanization, pollution, climate change, poor management, etc. all poses serious risk for water scarcity and worse water quality.
THE PROPERTIES OF WATER
- high surface tension
- capillarity
- high specific heat
- high heat of evaporation
- maximum density in the liquid state
- excellent solvent
Understanding the water properties is the first step for a good management of water resource.
Some of these properties can be explained with easy examples to the children.
Let’s start with capillarity and surface tension and later we move on to water temperature, pH and salt content.
Introduce the concept of surface tension.
When you turn on a water faucet so that only a small stream comes out, the water starts out as cylindrical column, and ends up as droplets. Have you noticed this? Why do the water do that? Why doesn’t the liquid stay in a stream? And why does it form round drops—why doesn’t it break into little cubes instead?
Try to collect answers from the children. If a student says that it is “easier” for the water to form spherical drops instead of cubes, encourage them to think about why it is easier
Let’s know focus on a lake and on the insects that very often you can see laying on the water surface. These insects are called water striders.

How do you think they got their name? How do they stay on the water surface?
One striking and interesting property of water is surface tension. The intermolecular forces cause the water molecules to be attracted to each other. These forces are known as “cohesive” forces. In the body of the water, a molecule is surrounded by molecules in all directions, so the attractive forces cancel, and the molecule feels no overall force. However, on the surface of the interface between the water and air, a molecule in the water feels the attractive forces of the other molecules within the water, but none from outside. This causes the outer layer of the water to act like a stretched membrane and minimize the surface area. Just as in a filled balloon, “tension” exists in this elastic surface, and energy is stored in this surface, just like elastic energy is stored in the rubber of the balloon. To minimize the stretching of the skin and lower the amount of energy in tension on the surface, the water molecules move to create the least surface area possible. It is for this reason that falling water drops form spheres—it allows for the least amount of surface area for a given volume of liquid.
The surface tension can be “seen” as a sort of membrane on the top surface of the water. Water striders are able to walk on top of water due to a combination of several factors. Water striders use the high surface tension of water and the long, hydrophobic legs to help them stay above water. Gerridae species use this surface tension to their advantage through their highly adapted legs and distributed weight.
In addition to the cohesion forces due to the intermolecular attraction, water exhibits also adhesion forces. Adhesive force is the force between the water molecules and the walls of the vessels. Capillary action or capillarity is caused when the adhesive force is stronger than the cohesive force. Capillary action is a process during which a liquid, like water, moves up something solid, like a tube, or into a material with a lot of small holes.
Nice and easy experiments on capillarity, surface tension and the effects due to pollutants.
Draw some flowers on light cardboard. You can make them in different sizes and with different colors to make the experiment cuter. Cut out the flowers and fold the petals to close them (you will find a template in the kit but you can use whatever you wish)

Take the water temperature sensor and the electronic board.
The water temperature sensor consists of a sealed metal housing and an internal temperature sensor, as shown below: when we put the water temperature sensor in the water, the temperature of the water is transmitted to the internal temperature sensor through the metal thermal conductor, which causes the sensor value to change. That’s how the water temperature sensor works.
Let’s start to use our board to read the water temperature.

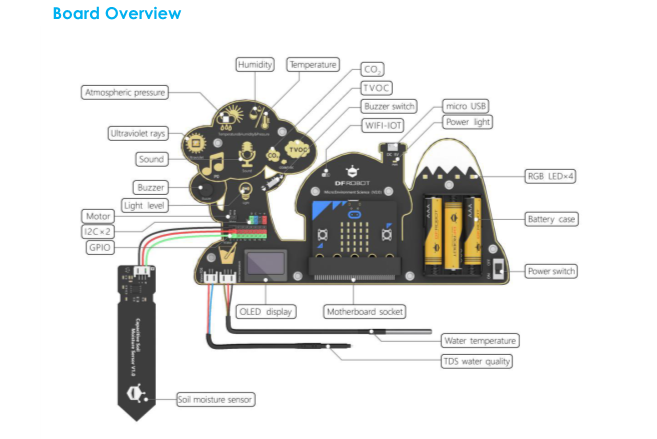
Plug the water sensor connector into the board white connector where water is printed on.
Import the file “microbit-Water_Celsius-F.hex”
It cleans up the oled, read the water temperature and print on the OLED screen both in Celsius and Fahrenheit degrees.
You may wish to add also the measurement of the environmental temperature to compare with the water one.
Are the measurements consistent each other? Do you notice any difference between the two systems?
pH
The substances we use unconsciously every day can be acidic or basic: what’s the difference?
Acids and bases are substances that neutralize each other and that we often encounter in everyday life. If we think of the term acid, something with a sharp, pungent, sour flavor comes to mind like a lemon, like certain carbonated drinks, like kiwi or orange juice. Some acids are commonly used in industrial processes: examples are hydrochloric (used for insecticides) and sulfuric (used for dyes and fertilizers). Even our body hosts very acidic environments: one of these is the inside of the stomach!
Bases are often part of household cleaning products: sodium bicarbonate, ammonia, bleach, caustic soda used to unclog clogged pipes and sinks are basic substances… Under normal conditions even human blood is slightly basic.
If finding acids and bases in everyday life is very easy, giving them a definition is a challenge that has puzzled several generations of scientists. The first was, in 1600, the British chemist Robert Boyle, who observed the objective characteristics of acids and bases: he noticed that the former corroded metals and had a sour taste, while the latter had a bitter taste and were slimy to the touch. Salts and water are obtained from the reaction between acids and bases (called neutralization). This is the reason for the term “base”: to obtain the salts, you will also need a starting base!
However, it was the Swedish scientist Svante Arrhenius who developed, at the end of the nineteenth century, the first real theory on acids and bases: he said that an acid is a substance that releases hydrogen ions (H+) in water while a base releases OH- ions (hydroxide ions). However, Arrhenius’ solution had the limitation of being only good for aqueous solutions. A more general one was needed, which would also apply to those substances for which it is not practical to evaluate their behavior in water. The Danish chemist Johannes Bronsted and his English colleague Thomas M. Lowry thought about it in 1922: according to the two scientists, an acid is a substance capable of donating H+ ions (protons), a base is a substance capable of accepting H+ ions (protons).
The pH of water is the measurement of the concentration of acids or bases in an aqueous solution, or, in other words, the measurement of the acidity of a solution. A solution is said to be “acidic” when its pH is between 0 and 7, “neutral” when the pH is equal to 7 and “basic” with values between 7 and 14.
The EU legislation on the potability of water intended for human consumption (requires that the pH value of water supplied by public aqueducts is within the range 6.5-9.5. This range guarantees ideal organoleptic characteristics, i.e. pleasant flavour, transparency and almost imperceptible odor and, at the same time, allows for optimal resistance to bacterial contamination.
The main methods for measuring pH are colorimetric, i.e. “tornasole paper (litmus paper)” and use of liquid reagents or tablets. Alternatively, the use of specific instrumentation is envisaged, i.e. a pH meter (measurement with electrode probe).
We will use tornasole paper for our measurements which are comprised in the kit.
Select several different water samples (you can use the same as before) and immerse in each of them a tornasole strip for few seconds. Let them dry up a bit and then compare with the color scale to understand the value of the ph.
The tornasole paper measurement provide a quali-quantitive estimate of the pH. For a more precise measurement you need to use a ph-meter sensor which however is not included in the kit and is out of the scope of this project.
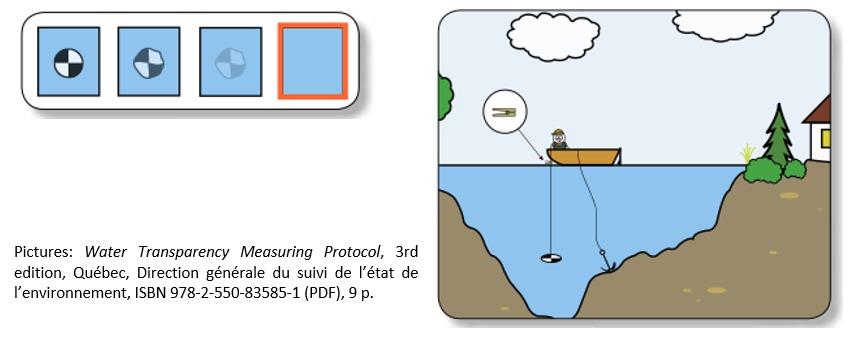
Transparency
Water transparency is directly linked to the property of water to transmit light. For small sample of water, you can just estimate water transparency by qualitative estimation looking at any possible suspended material.
For large water quantity the transparency may be estimated by an old and inexpensive system called “Secchi disk”. The Secchi disk (or Secchi disc), was originally created in 1865 by Angelo Secchi. Nowadays Secchi disk are realized by a black-and-white circular disk 20 cm in diameter mounted on a line and lowered slowly down in the water. The depth at which the disk is no longer visible is taken as a measure of the transparency of the water.

You may wish to build up one Secchi disk in your class and leave to the student for summer holidays.
You can use an old CD and color it black and white.
Introduction of TDS Water Quality: water as a solvent.
We learned that our drinking water resources are very limited, we understand water pollution, we know how to save water, protect water resources and so on. So now let’s learn about water quality measurements. To begin with, let’s look at a noun: TDS. TDS is an important
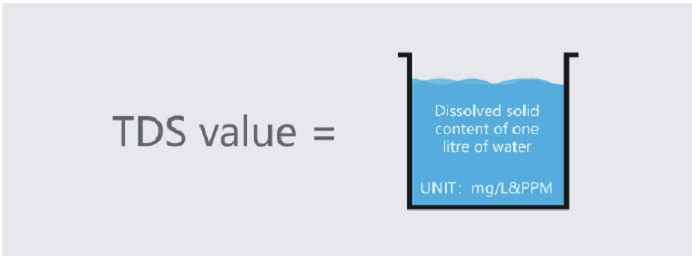
parameter that affects water quality.
What is the TDS value?
The TDS value refers to the total dissolved solid, also known as the total amount of soluble solids, measured in mg/L. It shows how many milligrams of soluble solids are dissolved in 1L of water. The higher the TDS value, the more dissolved matter is contained in the water, so we can easily say that the TDS value partly reflects the purity of the water. The lower the TDS value, the higher the water quality, the higher the TDS value, the more soluble solids contained in the water. Please pay attention: liquid with high TDS values are not necessarily harmful.
For example, for the water inside the river, TDS value is about 400. And tap water is about 100, bottled pure water is about 10, while the TDS value of juice is 500. From the values above, the purity of bottled pure water is very high, impurities are very small. The TDS value of fruit juice is 500, but it is harmless to humans.
But TDS is not the only criterion for determining water quality. TDS can only measure conductive substances in water, but cannot detect bacteria, viruses, and other substances.
Using the TDS values, it is possible to classify water into:
Fresh water: less than 500 mg/l or TDS = 500 ppm.
Brackish water: 500 to 30,000 mg/l or TDS = 500 to 30,000 ppm
Saline water: 30,000 to 40,000 mg/l or TDS = 30,000 to 40,000 ppm
Hypersaline: greater than 40,000 mg/l or TDS greater than 40,000 ppm.
Household tap water must have a TDS of less than 500ppm.
Let’s move to the measurement.
Connect the TDS probe to the electronic board. Leave connected or connect also the water temperature probe (as in the previous example)
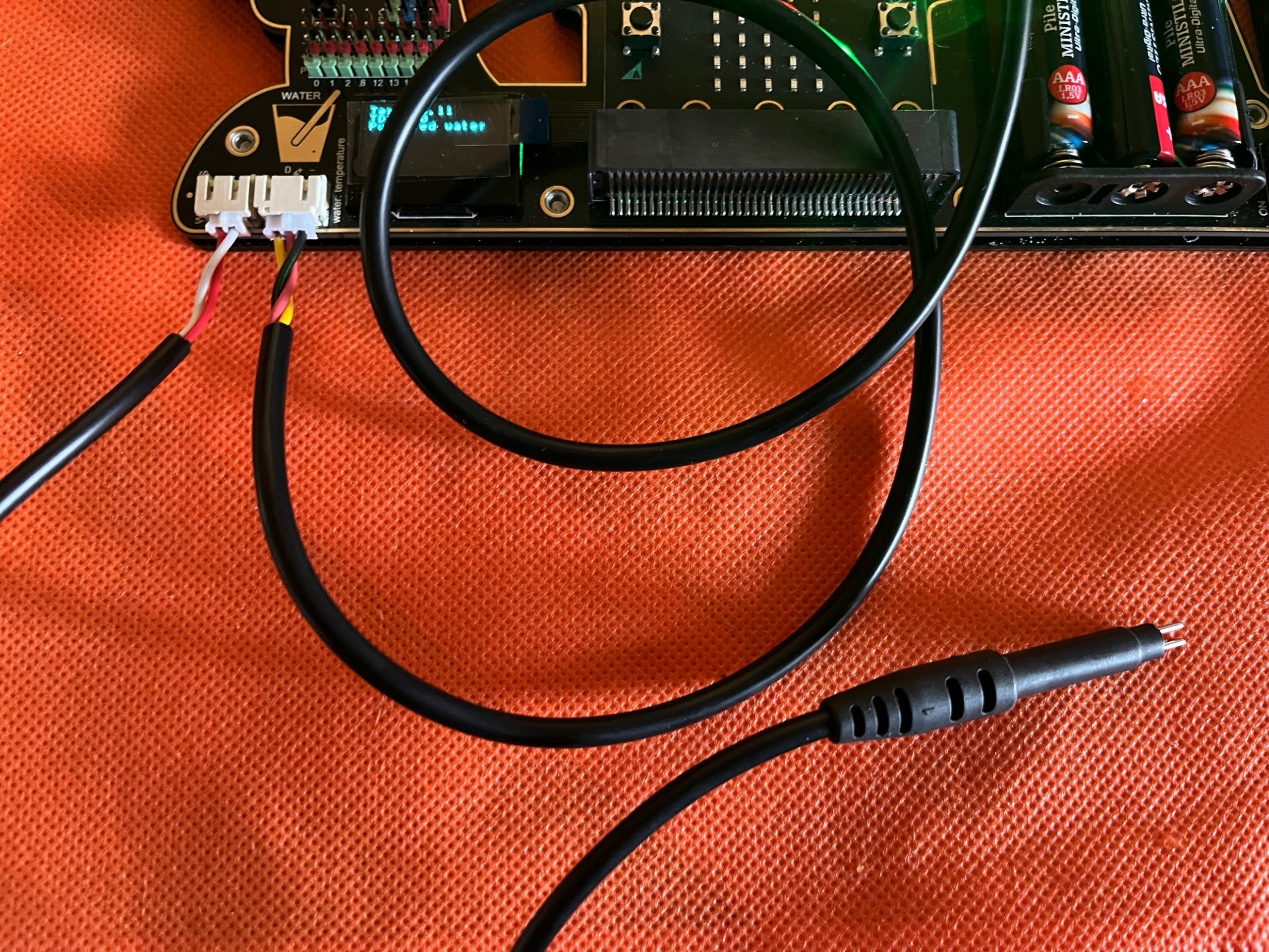
Import the file microbit-TDS01_Temp.hex from our repository: the code will enable you to measure both the water temperature and the TDS value. DOWNLOAD on the board in the usual way.
The code enables you to measure the TDS and water temperature.
According to the TDS value in the OLED you will find also a classification:
TDS<150 Pure water – Green light in the board
150<TDS<550 Tap water – Yellow light in the board
TDS>550 warning: Too high residuals in the water – Red Light in the board
Use all the information we learned to perform water quality measure parameters.
Select few samples of water:
- Mineral water (should be the lowest in TDS)
- Tap water
- Some soft drink or juice
- Prepare some solution of salt and water
- Etc.
Complete the following table
Soil and its properties
What is soil
Soil is the topmost layer of the Earth. It derives from the alteration of rocks, i.e. from their disintegration and transformation by air, water and living organisms.

A soil is always made up of three components.
- solid component;
- water, with dissolved mineral salts;
On average, the solid component occupies half of the volume of a soil, while the other half is made up of water and air.
Soil properties
Porosity is the set of tiny empty spaces, called pores, present between the solid particles of the soil.
Permeability is the ability of the soil to let water pass through it. It increases with the size and number of pores in the soil.
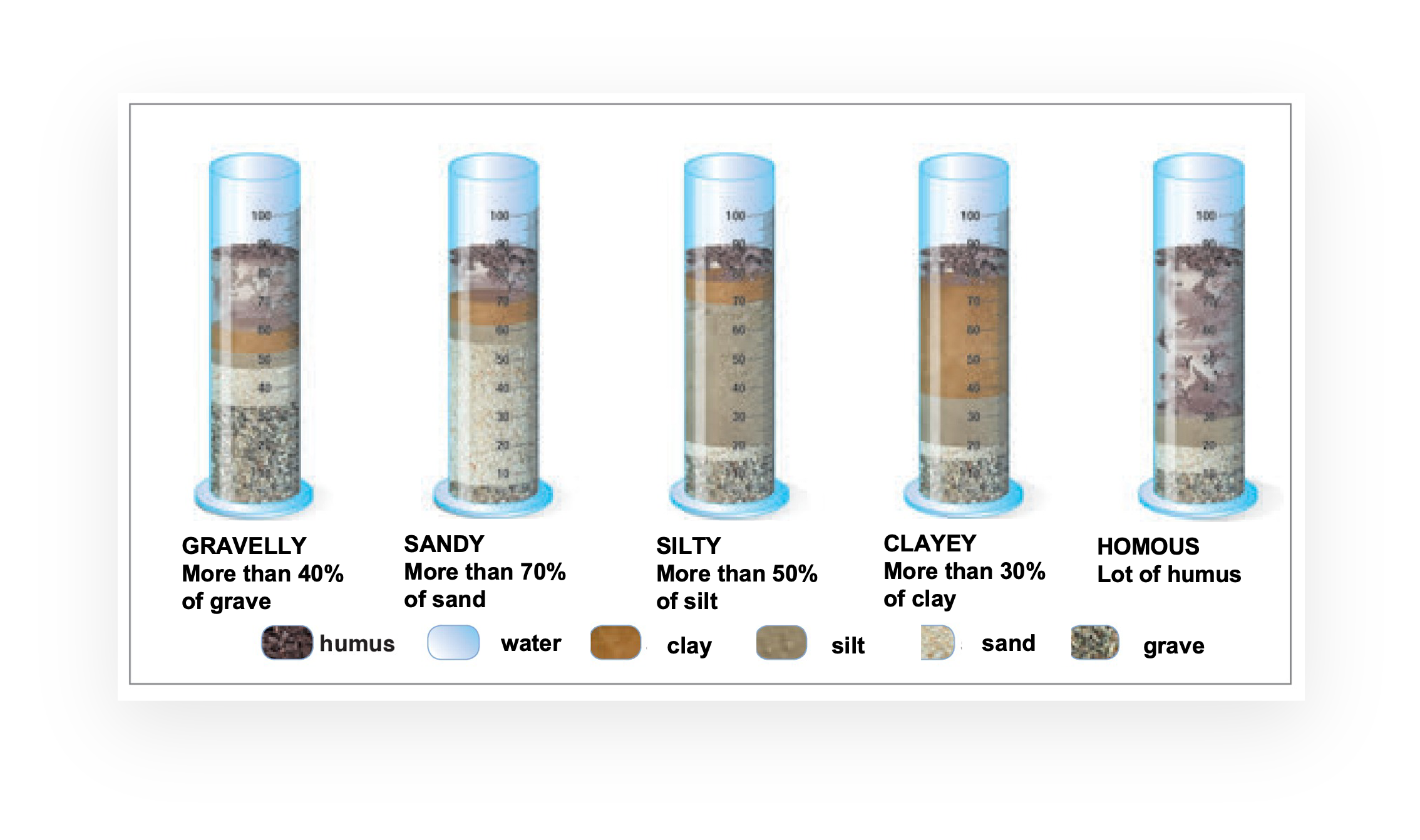
A physical characteristic of the soil is the texture, or grain, which indicates which type of granules are predominantly present: in fact, it may happen that a soil is made up of a large quantity of sand granules and little clay or that it contains a lot of humus and little gravel and so on. To measure how much gravel, sand, silt, clay and humus there are in a soil, it is necessary to mix a sample of the soil and let it settle at the bottom of the container so that the various components separate. The technique to use is sedimentation. The percentage distribution of the various types of granules allows soils to be classified as: gravelly, sandy, silty, clayey, humous.
An easy qualitative experiment to understand soil texture:
Take several containers and put different kinds of soils, leave for few hours (better a day) and look at them later on
Which kind of composition the soil samples have?
Permeability is also linked to the other physical characteristics of the soil. You will have noticed that, after the rain, the water disappears quickly in some areas, in others it stagnates and forms puddles on the surface. Why?
In the illustrated experiment the same quantity of water is poured on the same quantity of sand, clay and potting soil.
For each sample, the quantity of filtered water collected in the container is measured and the time taken by the water to filter is recorded by reading it on the chronometer. The results demonstrate that sand lets water pass faster, clay retains more, potting soil has a mixed composition and therefore has intermediate characteristics between the other two samples. A soil that allows water to pass through it easily and in a short time is defined as permeable.

Permeability is the ability of a soil to let water pass through it and depends on the porosity: the greater the porosity, the greater the permeability. Gravelly and sandy soils are permeable, while clayey soils are impervious.
Closely linked to these two characteristics is also the ability to retain water: if a soil is waterproof, it will remain soaked for longer, while if it is permeable it will dry easily and quickly.
Soil and Life
Soil is a very important natural resource, essential for life on Earth
The main agricultural processes are two:
- ploughing, which serves to break up the clods in depth to improve porosity and mixing with fertilizers and air;
- controlled irrigation, to give the soil the right amount of water, even with the arrangement of drainage channels and furrows for the flow of water.
One of the most effective cultivation techniques, developed since the Middle Ages, is crop rotation. In the three-year rotation the fields are divided into three strips in which three different crops are sown which alternate each year.
Plant life depends on the physical characteristics of the soil. Good soil must be porous and permeable, but not too much, to prevent it from drying out completely, and retain water, but not too much, to prevent the roots from rotting: that is, it must have the different characteristics in the right balance. Some vegetables, however, such as potatoes, carrots and succulents, live well in sandy and permeable soil; others, such as rice, wheat and corn, prefer clayey and impermeable soils: it depends on the type of root and how the plant itself retains water. Even the chemical characteristics of a soil are important for plants: olive trees, vines and legumes are suitable for living in calcareous soils; the blueberry, the rhododendron, the chestnut live well in siliceous soils.
Water in soil
The amount of water in soil (humidity or moisture of the soil) is one of the most important parameters to guarantee safe life of plants
The optimal humidity depends on the type of plants but in general a good humidity of the soil should range between 70-90 %.
Let’s measure together the humidity of the soil with our electronic board. In order to do that we need to plug the soil moisture sensor in out electronic board.
Connect the connector of the sensor to the second line of pins in the board. Pay attention to the polarity. The black cable goes to the black pin.
Before being able to read reasonable results, we must calibrate the sensor in dry environment (humidity 0%) and in water (humidity 100%). Perform this measurement before using the board with the students.

Import the file microbit-Soil_calibration.hex from our repository: the code will enable you to calibrate the sensor. DOWNLOAD on the board in the usual way.
Try to first measure the humidity of a completely dry soil and annotate the number, repeat the measurement by measuring the humidity of water and annotate this second number. In my set-up the dry value corresponds to 802 while the water one to 400. Your values should be in the same range, but they may differ a bit.
Import the file microbit-Soil_calibrated.hex from our repository: the code will enable you to read the water temperature using the appropriate sensor and the soil moisture. DOWNLOAD on the board in the usual way.
Program description: the code cleans up the OLED screen and then ask the electronic board to provide the value of the water temperature and soil moisture. You need to change the calibration values according to your previous measurement. Modify the program and put your values. In the example program, we map 400 to 10, 802 to 0. In the actual test, the humidity is 0 when reaches the lowest, and the highest humidity is 10.
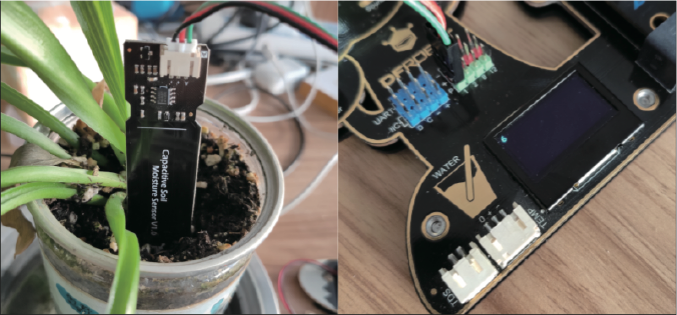
Now you are ready to use it with the students.
Take several different soil mixtures and plug the sensor in the different samples. Read the soil moisture in %. You can do directly taking several different plants. Typical ideal moisture should be around 70%.
Without our atmosphere, life on Earth would not exist. Our atmosphere is a complex system that interacts with the hydrosphere, geosphere and the biosphere. For example, pollutants can move from the hydrosphere into the atmosphere; from there they can be transported and impact the biosphere. None of these systems are isolated. Our atmosphere is ~21% oxygen and ~78% nitrogen; the remaining 1% is considered “trace gases” and this includes everything else—from carbon dioxide to the noble gases like argon. When we think of air quality, we typically think of the air we are breathing and whether or not it is safe.
Poor air quality can negatively affect human and environmental health. In humans, poor air quality can lead to a multitude of problems that include respiratory and cardiovascular diseases. We tend to think first of asthma and respiratory problems, but some particles are so small that they can enter the blood stream through the lungs and cause inflammation leading to issues beyond our breathing. In plants, poor air quality can also cause diseases that can result in crop loss. In addition to human and environmental health, many pollutants that we worry about are greenhouse gases and contribute to climate change.
Air pollution
Pollutants come from both natural and anthropogenic (human-made) sources.
- Combustion is a major source of pollution; two examples are vehicles and forest fires, however, both of these sources are burning different fuel types in different conditions, meaning the emission profiles will likely be quite different (potential pollutants: CO2, NOx, CO, VOCs, PM).
- Compound volatilization (VOCs see later) also results in emissions. Industrial activity is a good example of when VOCs might be regulated to both prevent health impacts to workers and emissions into the atmosphere.
- Physical generation includes both human-made and natural occurrences, but the result is always dust or particulate matter, whether it is a desert wind storm, or a car on a dirt road, the result is more particulates in the atmosphere.
Carbon dioxide is a colorless and odorless gas at room temperature. It is denser than air and can dissolve in water. The chemical formula is CO2 and it is one of the main components of the air.
CO2 is always present in our atmosphere however too much carbon dioxide in the air can cause breathing difficulties and even carbon dioxide poisoning.
We will see a couple of easy experiments in which CO2 is generated in chemical reactions.
- Blow out a candle: the formation of CO2 during combustion
This is an easy experiment to realize to demonstrate that a gaseous substance, if it becomes too abundant in the air, can become harmful, and therefore polluting. This type of substance can originate during combustion or chemical reactions of various kinds that occur in the environment.
Material
- 1 warming candle
- Matches or lighter
- 1 bowl
- Vinegar
- Sodium bicarbonate
We wish to show that a polluting substance can be produced during combustion or chemical reactions that occur in the environment.
- Pour some vinegar into a container (wide but not too tall).
- Place the candle on the bottom.
- Light the candle
You don’t see anything strange.
Now
- Pour a spoonful of baking soda into the container
You will see that the candle will go out.

Vinegar and baking soda react and form CO2
The production of CO2 stopped the burning reaction of the candle. Carbon dioxide is a pollutant and can be formed in large quantities during combustion and chemical reactions.
- Inflate a balloon: the formation of CO2
What you need: bottle (glass, plastic), bicarbonate of soda, vinegar, balloon
- Place a small amount of bicarbonate of soda at the bottom of the bottle.
- Pour some vinegar into the bottle
- Quickly put a balloon on the neck of the bottle
You will see the ballon inflating. Why?
➢ Explanation: a chemical reaction occurs between vinegar and bicarbonate which produces CO2.
Like all gases, carbon dioxide also expands, inflating the balloon to occupy the space inside it.

- Understand the effect of acid rain…
What you need:
A small dish (plastic or glass), chalk, lemon juice, coke, detergent, concentrated citric acid, plastic pipettes
- Break-up a piece of chalk on the dish
- Pour a few drops of the various liquid substances (which have different level of acidity – you can also test with the litmus paper) onto each piece pf chalk
What happens?

Acidic substances dissolve the chalks more or less quickly. By analogy, acid rain is capable of crumbling the material of which buildings, monuments, etc. are made.
- The greenhouse effect
Purpose of the experiment is to understand the role of greenhouse gases: they are necessary to guarantee life on earth but if they are in excessive quantities they are harmful.
Material needed:
- 2 identical glasses
- Water
- 2 thermometers
- 1 plastic bag
- 1 elastic
- sunlight or a lamp
The sun’s rays heat the Earth. Part of the heat is absorbed by the ground, while part is returned to space. Some gases in the atmosphere, such as carbon dioxide, retain this heat. They are called “greenhouse gases”. This natural phenomenon guarantees an optimal temperature on Earth. Otherwise, it would be -18°C and life on Earth would not be possible!
The experiment
1) Fill two plastic cups with the same amount of water.
2) Place a thermometer in each of the glasses.
3) Secure a plastic bag to one of the two glasses with an elastic band.
4) Turn on a lamp or place the two glasses in sunlight to illuminate them.
5) Wait 2-3 hours.
Observe the two cups. What’s happened?
The water level in the glass without the bag is lower than that in the glass covered by the bag, on the contrary the temperature of the latter is higher.
After a few hours in the sun, you can observe that some of the water in the bagless glass has evaporated. In the second glass, the one with the plastic bag, the water could not escape. The level is the same, and the water is warmer than at the beginning. The temperature is higher than that of a glass left in the open air: it is the phenomenon of the greenhouse effect!
Greenhouse gases are necessary to ensure life on Earth. But if their percentage increases too much, they retain too much heat and life on the planet will become increasingly difficult. There are many sources of greenhouse gases: among these are cars and factories. It is therefore necessary to pay attention to the way we live and the products we use.
- The consequences of pollution on plants
This experiment is meant to show what the consequences of air pollution are on living beings.
Material
- 2 identical plants
- Dust
- Aerosols (hairspray, spray deodorants…)
- Cerini (to be used in the presence of an adult)
- Cigarettes (Attention: it is the teacher who must procure the cigarettes and must keep them away from the students)
We wish to demonstrate how the plant in the experiment is damaged by different atmospheric pollution while the second plant kept in a “normal” environment does not undergo visible changes.
1) Take two identical plants.
Arrange them at such a distance that they receive the same amount of light. Water them equally.
2) Subject the plant being tested to various polluting substances: ash, cigarette smoke and aerosols.
3) Repeat this operation 3 times a day for 2 weeks. Record daily observations (appearance of leaves, size, general appearance of the plant under the experiment and of the “normal” plant).
4) Water the two plants in the same way.
5) At the end of two weeks, the appearance of the plant object of the experiment has changed.
The experiment is concluded and the objective has been partially achieved given that the appearance of the plant subject to the experiment has changed. In fact, small yellow spots appeared on the leaves of the plant subjected to the polluting substances. Furthermore, the plant under study lost more leaves than the other. However, its size has not changed. Perhaps it would be worth extending the duration of the experiment so as to be able to observe further changes linked to the time of exposure to pollutants. Pollution is not only generated by industries. Cars, the production of electricity, the use of paints, pesticides and many chemical products also pollute.
We can measure CO2 concentration also by using our electronic board.
In this table you may find CO2 concentration (ppm) reference values:
Measurement of CO2 concentration
Import the file CO2.hex from our repository.
This program will read the value of carbon dioxide and display it on the OLED. When the value is below 500, the RGB light shows green; 500-1000 shows yellow; 1000-2500 shows orange; 2500-5000 shows red; above 5000 shows purple. Because the gas we breathe out also contains a lot of carbon dioxide, we can blow to the sensor to observe the significant change of the value.
Volatile organic compounds (VOCs) are a group of compounds with high vapor pressure and low water solubility. In other words, these substances won’t easily bind to themselves (volatile) or dissolve in water (organic). VOCs are emitted as gasses from everyday products such as building materials, maintenance equipment, and custodial products. Many VOCs are harmful to human health, especially over the long term.
Much like particulate, the term “VOC” doesn’t refer to a specific substance; instead, it refers to a group of substances that exhibit similar chemical properties. There are thousands of these substances, with some examples commonly found in buildings including:
- Benzene – found in tobacco smoke, paint thinner, deodorizers, air fresheners, furniture polish
- Formaldehyde – found in disinfectants, furniture upholstery, carpets, plywood
- Ethylene glycol – found in cleaning agents, personal care products, perfumes
- Methylene chloride – found in spot removers, dry cleaned clothes, fabric cleaners, commercial solvents, air conditioner refrigerant
- Tetrachloroethylene – used in solvents, dry cleaning, paint strippers
- Toluene – used in paint, metal cleaners, adhesives
When measuring the amount of VOCs in your home, you will often come across the term TVOC, or total volatile organic compounds. Just what does TVOC stand for? What is TVOC?
Total volatile organic compounds (TVOC) is a group of VOCs used to represent the entire pool of pollutants. TVOC refers to the organic compounds whose saturated vapor pressure exceeds 133.32 Pa at room temperature. Its boiling point is between 50 to 250°C at room temperature, and it exists in the air in the form of evaporation. Its toxicity, irritation, carcinogenicity and special odor, will affect the skin and mucous membrane, and produce acute damage to human body. TVOC is measured in parts per billion (ppb) or milligrams per cubic meter (mg/m3).
TVOC reference values:
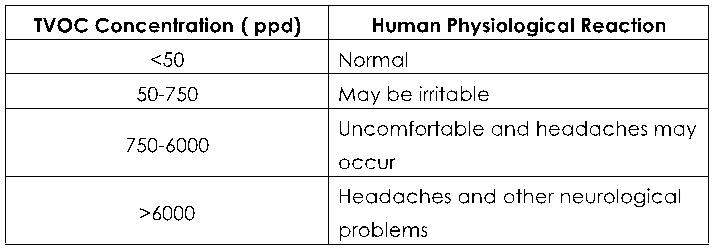
Measurement of TVOC concentration
Import the file microbit-TVOC.hex from our repository.
This program will read the value of TVOC and if it is greater than 750 it will light red on the first led.
Conclusions
Environmental protection is not only the public welfare but also the responsibility of everyone, no matter what we do, as large as afforestation, as small as garbage classification. Everyone should make effort to protect the environment. A proper knowledge of environment and the most common pollutants is mandatory to raise awareness about human impact on our planet and to create future citizens who are more aware and respectful of nature. This kit gives the possibility to measure the main environmental parameters and pollutants by using poor materials which are available in everyday life. The use of the electronic board is an additional opportunity to familiarize with IoT approach and gives the opportunity to develop digital skills and a multidisciplinary approach at school.
References
Tutorial of Environment Science Expansion Board for micro:bit -V2.0 Based on MakeCode
(SKU: MBT0034) – www.DFRobot.com
HUB Scuola Mondadori Education
NASA Ocean Science Division
NASA Earth Science Division
Water Transparency Measuring Protocol, 3rd edition, Québec, Direction générale du suivi de l’état de l’environnement, ISBN 978-2-550-83585-1 (PDF), 9 p.
Mondo Scienze B-La Terra, Bruna Negrino, Edizioni Il Capitello
Noi e l’aria, AtmoSud, ARPA Valle d’Aosta e ARPA Piemonte www.noielaria.it
APPENDIX
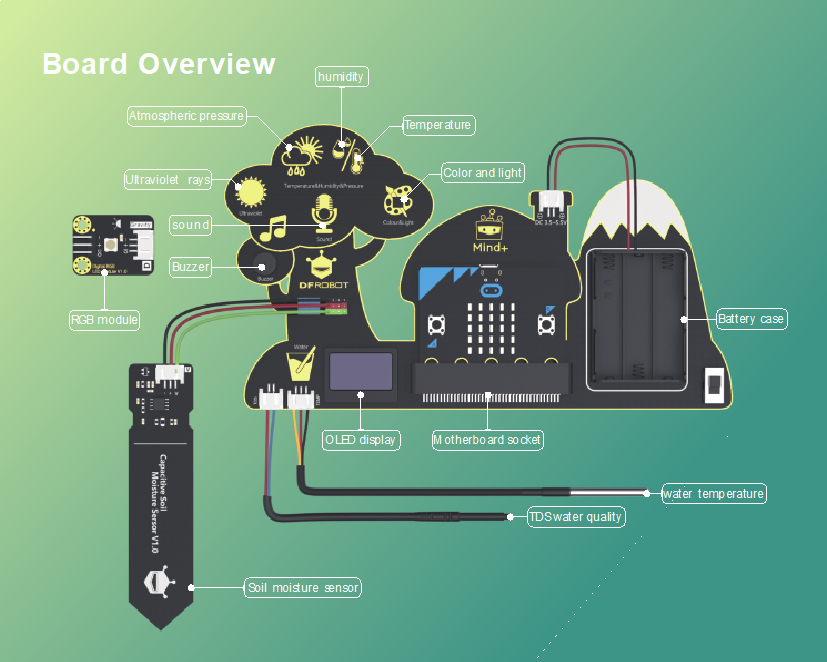
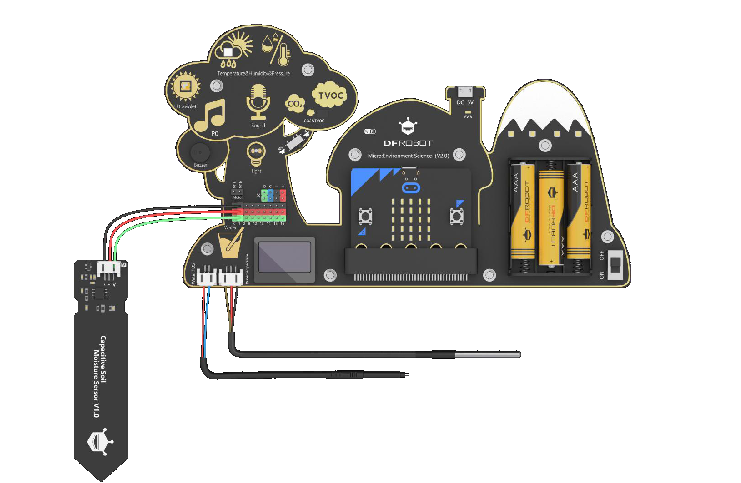
1 Electronic Board: Environment Science Expansion Board for micro:bit -V2.0 (SKU: MBT0034)
1 MicroBit v2.X card
USB cable
3AAA Battery (not needed if you connect the card to the computer)

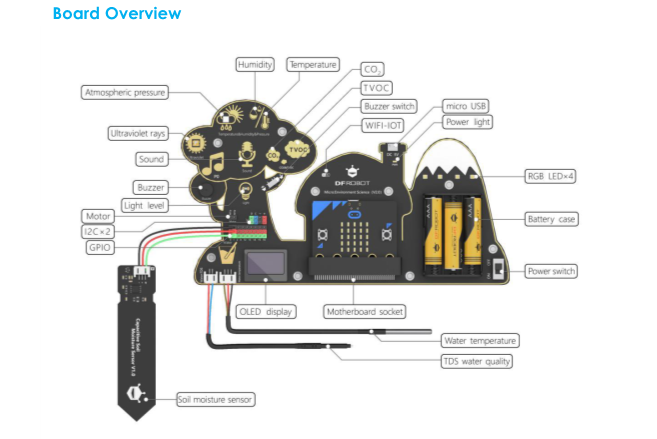
This micro:bit-based expansion board allows students to measure environmental conditions for scientific experiments by using rich on-board sensors. It aims to provide a platform for students to learn theory with practices and bring science education closer to daily life!
Integrated sensors include temperature sensor, humidity sensor, air pressure sensor, sound sensor, UV sensor, light sensor, water temperature sensor, and a TDS (Total Dissolved Solids) water quality sensor, soil moisture sensor, etc.
The specific characteristic of each sensor is reported in the following
BME280 Environment Sensor
Operating Current: 2mA
Operating Temperature: -40 oC – +85 oC
Temperature Measuring Range: -40 oC – +85 oC; Resolution 0.1 oC,Deviation ±0.5 oC
Humidity Measuring Range: 0~100%RH, Resolution 0.1%RH,Deviation±2%RH
Response Time of Humidity Measurement: 1S
Atmospheric Pressure Measuring Range: 300~1100hPa
Waterproof Temperature Sensor
Temperature Display Range: -10 oC – +85 oC (Deviation±0.5 oC)
Operating Temperature Range: -55 oC – 125 oC
Query Time: less than 750ms
TDS Water Quality Sensor
The TDS probe should not be used in water above 55°C.
The TDS probe should not be placed too close to the edge of the container,
as this will affect the accuracy.
CCS811 Air Quality Sensor
Operating Temperature Range: -40°C~85°C
Operating Humidity Range: 10%RH~95%RH
CO2 Measuring Range: 400ppm~8000ppm
TVOC Measuring Range: 0ppb~1100ppb
Capacitive Soil Humidity Sensor
Operating Voltage: 3.3V-5.5V DC
Output Voltage: 0-3.0V
DC Connector: PH2.0-3P
RGB Light
RGB Light Model: WS2812
Port: P15
Light Sensor
Output Date Type: analog value
Data Range: 0-1023
ML8511 UV Sensor
Operating Temperature: -20°C~70°C
Sensitive Area: UV-A, UV-B
Sensitivity Wavelength: 280-390nm
Buzzer
Dimension: 9mm in diameter
Software repository
https://drive.google.com/drive/folders/1He2CAtqvvJDPl7HI7aVEE3QqQ-7zIZKg?usp=share_link
Instructions: let’s keep the environmental board and the micro:bit board. Insert the micro:bit board in the appropriate slot and connect the micro:bit board to your computer using the USB cable.
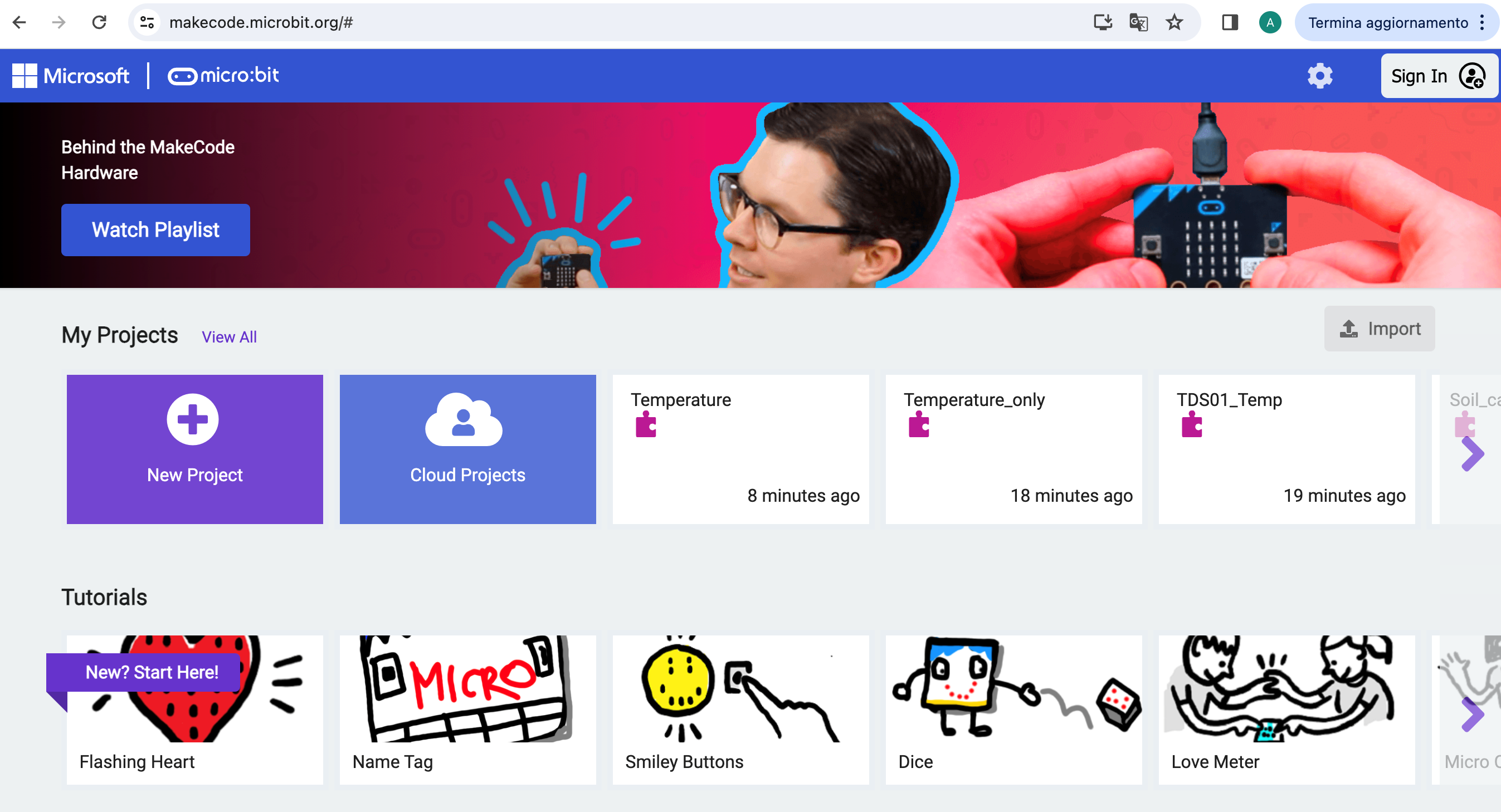
Open a browser (we strongly suggest to use Chrome) and go to the makecode site
https://makecode.microbit.org/
Click on the import button on the left, select “import File” and upload from the repository:
….
The file named, i.e., microbit-Temperature.hex
On the webpage you will see
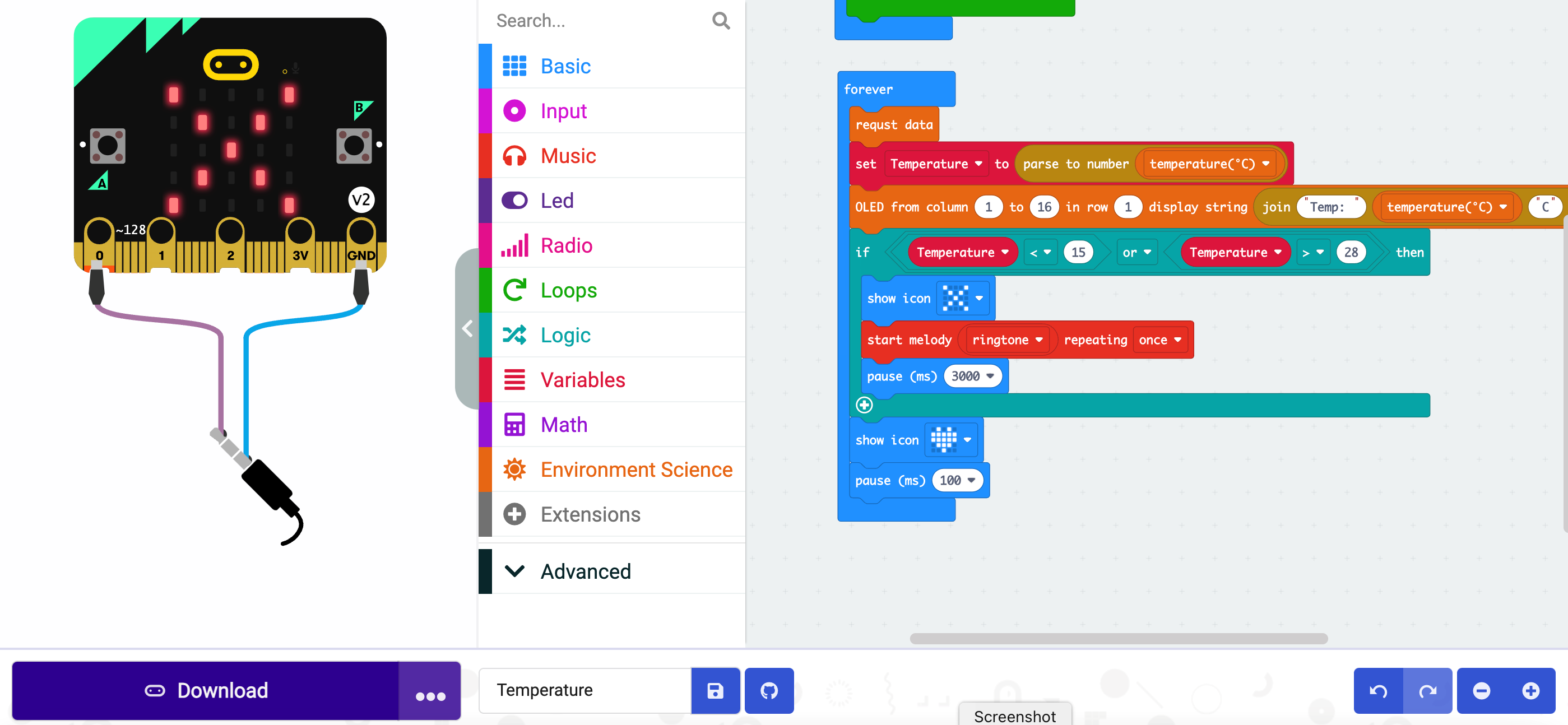
In order to transfer the code to the electronic board, the first step to do is to clic on
DOWNLOAD (bottom left)
If it is the first time that you connect the board to the PC it will ask you to pair the PC and the board, otherwise it will do automatically.